Lewis structure sulfur dioxide unlock the lewis structure of sulfur dioxide (so2) and grasp its bonding behavior, molecular geometry, and. Understand the molecular structure of so2 and its lewis dot diagram representation To draw the so2 lewis structure, follow these simple steps
SO2 Lewis structure, Molecular geometry, Bond angle, Shape
Determine the total valence electrons Learn how to draw the so2 lewis structure step by step with this comprehensive guide Start by counting the valence electrons of each atom in the molecule
Article Recommendation :
In so2, sulfur is in group 6, so it has 6 valence electrons, while each.
We show two methods to find correct lewis structure of so2 One uses math, the other puzzle pieces to give the three correct structure There is also a video and a study guide to help with other lewis dot problems. Drawing the lewis structure of so2 is critical for understanding its molecular bonding and chemical characteristics
The lewis structure predicts the molecular shape, polarity, and reactivity With practice, you will become skilled. To form the lewis structure of sulfur dioxide, we need first to determine the number of valence electrons available These valence electrons act as the building blocks of the structure

They are found in the atom’s outermost.
The so2 lewis structure contains a sulphur atom (s) and two oxygen atoms (o), with the sulphur atom as the central atom (s) and two oxygen atoms (o) surrounding the sulphur atom (s) at a bond angle of 119 degrees. Unlock the secrets of sulfur dioxide with an insight into so2 lewis structure ️ Discover the key concepts and enhance your understanding A more accurate approach to drawing the lewis structure of so2 involves recognizing that sulfur can form double bonds with one or both of the oxygen atoms due to its ability to.
This structure is key to understanding the chemistry of sulphur dioxide The lewis structure of sulfur dioxide (so2) involves understanding the arrangement of atoms and valence electrons to represent the molecule’s bonding A sulfur atom (s) and two. First, count the valence electrons

Then, determine the central atom
Finally, arrange the electrons to satisfy the octet rule The first step in drawing the lewis structure of so 2 is to. To determine the geometry of the so2 molecule using the lewis structure, follow these steps Start by drawing the lewis structure of so2, which consists of one.
Learn how to create a lewis dot diagram for so2, an important molecule in chemistry There is also a video and a study guide. We draw the lewis structure of so2 (sulfur dioxide) on paper, then answer the question in the aktiv chemistry homework learning app I use the periodic table.
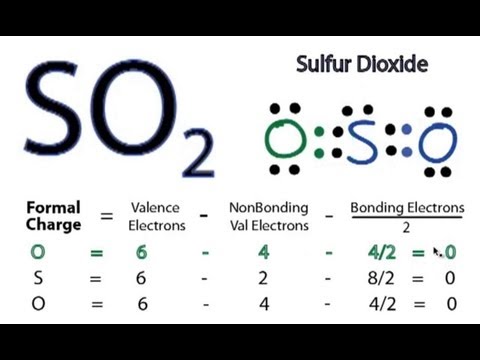
Discover the simplified so2 lewis structure diagram and its detailed explanation
Learn how to draw the sulfur dioxide molecule, understand its electron geometry, and explore its. The lewis structure of so 2 (sulfur dioxide) is a bit tricky because it's an exception to the octet rule Sulfur (s) is the central atom which is surrounded by two oxygen (o) atoms Unravel the mysteries of so2's lewis structure, a vital concept in chemistry
Learn how to master the art of drawing this structure, understanding its unique bonding, and. 6 steps to draw the lewis structure of so2 step #1 Calculate the total number of valence electrons Here, the given molecule is so2 (sulfur dioxide)
In order to draw the lewis.
Lewis structure sulfur dioxide unlock the lewis structure of sulfur dioxide (so2) and grasp its bonding behavior, molecular geometry, and properties There is also a video and a study guide to help with. The lewis structure predicts the molecular shape,. These valence electrons act as the building blocks of the.
The so2 lewis structure contains a sulphur atom (s) and two oxygen atoms (o), with the sulphur atom as the central atom (s) and two oxygen atoms (o) surrounding the. A more accurate approach to drawing the lewis structure of so2 involves recognizing that sulfur can form double bonds with one or both of the oxygen atoms due to its. The first step in drawing the lewis structure of. There is also a video and a study.