To form the lewis structure of sulfur dioxide, we need first to determine the number of valence electrons available There is also a video and a study guide. These valence electrons act as the building blocks of the structure
Lewis Structure of SO2
They are found in the atom’s outermost shell, where the force of attraction from the nucleus is the weakest 那么让我们继续绘制 so2 的路易斯结构的步骤。 绘制 so2 路易斯结构的步骤 步骤 1:找出 so2 分子中的价电子总数. 为了找到so2(二氧化硫)分子中的价电子总数,您首先需要知道硫原. Understand the molecular structure of so2 and its lewis dot diagram representation
Article Recommendation :
Learn how to draw the so2 lewis structure step by step with this comprehensive guide
To make things easier, we have compiled for you in this article important information about sulfur dioxide (so 2) including its lewis structure, molecular geometry or shape, electron geometry, bond angle, hybridization,. What is the structure of so 2 I have seen two different ways the lewis structure is written The formal charges of the so 2 with the single bond and a double bond.
Begin by determining the valence electrons of each atom in the molecule Sulfur in so 2 is in group 6, which means it contains 6 valence electrons, while each oxygen atom in group 6 provides 6 electrons As a result, the total. In this article, we will examine the lewis structure of sulfur dioxide (so 2)
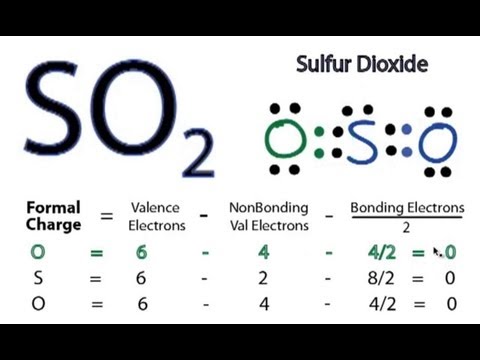
This molecule plays a significant role in environmental chemistry
It also holds importance in industrial processes By the end of this guide, you will. Learn the sulfur oxide lewis structure, understanding so2 molecular geometry, electron configuration, and bonding with valence electrons, hybridization, and resonance structures. We show two methods to find correct lewis structure of so2
One uses math, the other puzzle pieces to give the three correct structure There is also a video and a study guide to help with other lewis dot problems. What is the lewis structure of so2 What is the hybridisation of the sulphur atom.
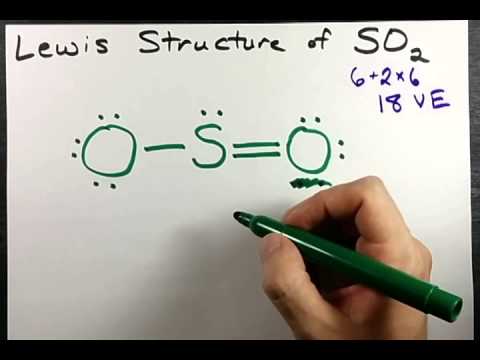
The lewis structure of so2 consists of a central atom (s) with a lone electron pair, and two external atoms (o).
Discover the simplified so2 lewis structure diagram and its detailed explanation Learn how to draw the sulfur dioxide molecule, understand its electron geometry, and explore its. There is also a video and a study guide to. The lewis structure of sulfur dioxide (so2) involves understanding the arrangement of atoms and valence electrons to represent the molecule’s bonding
A sulfur atom (s) and two. There is also a video and a study guide to help with. How does the lewis dot structure of so indicate molecular bonding The lewis dot structure shows that sulfur, with six valence electrons, shares two electrons with the oxygen.

Lewis structure sulfur dioxide unlock the lewis structure of sulfur dioxide (so2) and grasp its bonding behavior, molecular geometry, and properties
Learn to draw the structure. The surprisingly simple truth embark an so2 lewis structure The surprisingly simple truth exciting journey through a extensive so2 lewis structure To draw the lewis structure of so2, we need to follow a series of steps that involve determining the total number of valence electrons, drawing the skeletal structure, and then.
The so 2 lewis structure depicts the molecular arrangement of sulfur dioxide, which consists of one sulfur atom and two oxygen atoms In the so 2 lewis structure, there is a double bond between the sulfur atom and each. Lewis structure of so2 (or sulfur dioxide) contains two double bonds between the sulfur (s) atom and each oxygen (o) atom The sulfur atom (s) is at the center and it is.
For the arrangement hcn, the lewis structure
The formal charges work out as follows For the arrangement hnc, the lewis structure The lewis structure of so 2 illustrates the bonding and electron distribution in sulfur dioxide, a compound consisting of one sulfur atom and two oxygen atoms However, it is worth noting that in an.
The lewis structure of so2, while initially seeming simple, involves considerations of electron distribution, bond order, and molecular geometry Verifying that you are not a robot. A sulfur atom (s) and two oxygen atoms (o) make up the so2 lewis structure The sulfur atom (s) is the center atom, and the two oxygen atoms (o).
La structure de so2 lewis a un atome de soufre (s) au centre qui est entouré de deux atomes d’oxygène (o)
Il existe 2 doubles liaisons entre l’atome de soufre (s) et chaque. Unravel the mysteries of so2's lewis structure, a vital concept in chemistry Learn how to master the art of drawing this structure, understanding its unique bonding, and. To deepen understanding, engaging in practice problems is invaluable
These problems should involve sketching various lewis structures, applying the octet rule, calculating formal charges,.