We show two methods to find correct lewis structure of so2 About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket press copyright. One uses math, the other puzzle pieces to give the three correct structure
Lewis Structure of SO2
There is also a video and a study guide to help with other lewis dot problems. Do not draw double bonds unless they are needed for the central atom to obey the. Drawing the lewis structure for so2 is essential for understanding its molecular bonding and chemical properties
Article Recommendation :
Learn how to draw the lewis structure of so₂, understand its bonding, molecular shape, resonance, and electron distribution in simple steps.
Drawing the lewis structure of so2 is critical for understanding its molecular bonding and chemical characteristics The lewis structure predicts the molecular shape, polarity, and reactivity. Unlock the lewis structure of sulfur dioxide (so2) and grasp its bonding behavior, molecular geometry, and properties Learn to draw the structure and explore so2's polarity, bond angles, and more.
The lewis structure of so2, or sulfur dioxide, is a fundamental concept in chemistry that illustrates the molecular geometry and bonding between atoms in this compound. The lewis structure of sulfur dioxide (so2) involves understanding the arrangement of atoms and valence electrons to represent the molecule’s bonding A sulfur atom (s) and two. To form the lewis structure of sulfur dioxide, we need first to determine the number of valence electrons available
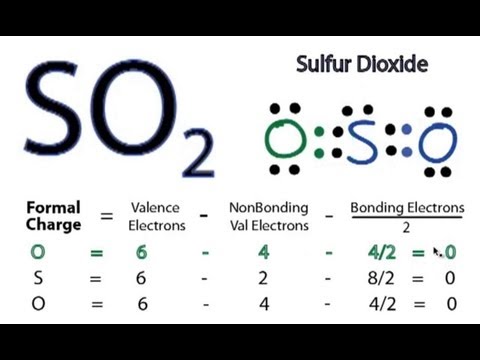
These valence electrons act as the building blocks of the structure
They are found in the atom’s outermost. The lewis structure for so 2 requires you to place more than 8 valence electrons on sulfur (s) You might think you've got the correct lewis structure for so 2 at first. Learn how to create a lewis dot diagram for so2, an important molecule in chemistry
Learn the so2 lewis structure, including sulfur dioxide molecular geometry, valence electrons, and bond angles, to understand its chemical properties and reactivity. We draw the lewis structure of so2 (sulfur dioxide) on paper, then answer the question in the aktiv chemistry homework learning app. With its distinctive shape and bond angles, so2 provides an excellent case study for students to delve into molecular geometry and electron distribution Join us as we dissect the lewis.

What is the structure of so 2
I have seen two different ways the lewis structure is written The formal charges of the so 2 with the single bond and a double bond is larger than the so 2 with. To determine the geometry of the so2 molecule using the lewis structure, follow these steps Start by drawing the lewis structure of so2, which consists of one sulfur.
Be sure to use the proper capitalization for all element symbols For the lewis structure of individual elements, use our valence electron calculator The calculator will generate the lewis structure for known isomers along with the bonds, ionic. Sulphur is sp2 hybridised and the lone pair of electrons of sulphur reduces the bond angle from 120° to 119°

There is also a video and a study guide to help with other lewis dot problems
There is also a video and a study guide. Click here:point_up_2:to get an answer to your question :writing_hand:what is the lewis structure for so2 d01e79 1 La struttura di lewis so2 ha un atomo di zolfo (s) al centro circondato da due atomi di ossigeno (o) Ci sono 2 doppi legami tra l’atomo di zolfo (s) e ciascun atomo di ossigeno (o).
This structure is key to understanding the chemistry of sulphur dioxide Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle Find more chemistry widgets in wolfram|alpha. Learn the steps to draw the lewis structure of so2 in just 1 minute.📌you can draw any lewis structures by following the simple steps mentioned in this artic.
The lewis diagram for so2 shows sulfur in the center with two oxygen atoms attached by double bonds
This represents the sharing of electrons between sulfur and oxygen,. The “best” lewis structure is one in which has the fewest formal charges The molecular formula of sulfur dioxide (so 2) shows that it has one sulfur (s) atom and two oxygen (o) atoms Sulfur and oxygen are located in group 16 of the periodic table.both have six valence.
The final lewis structure for so2 is as follows What is the lewis structure for so2 The lewis structure for so2 consists of a central sulfur atom bonded to two oxygen atoms The lewis dot structure for sulfur dioxide (so2) depicts its molecular structure and bonding
The sulfur atom (central entity) is bonded to two oxygen atoms (bonded entities),.
The lewis dot structure for sulfur dioxide (so2) consists of one sulfur atom bonded to two oxygen atoms The sulfur atom has six valence electrons, while each oxygen atom has six. Draw (on paper) a lewis structure for so2 and answer the following questions based on your drawing